Catalytic Methods
Highlights in Recent Catalytic Methods Development
Organophotocatalytic Functionalization of Unactivated Olefins
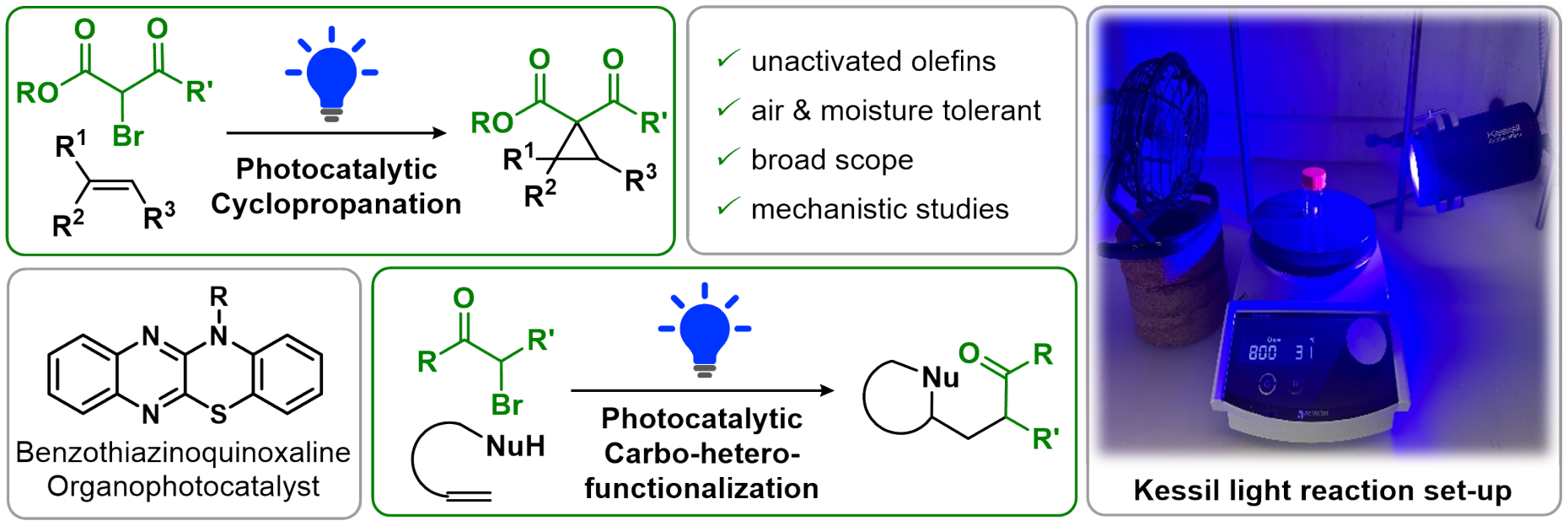
For more information, check out the following publication:
- external page D. M. Fischer, H. Lindner, W. M. Amberg, E.M. Carreira, J. Am. Chem. Soc. 2023, 145, 774-780.
- external page D. M. Fischer, M. Freis, W. M. Amberg, H. Lindner, E.M. Carreira, Chem. Sci. 2023, 14, 7256-7261.
- external page H. Lindner, W. M. Amberg, E.M. Carreira, J. Am. Chem. Soc. 2023, 145, 22347-22353.
Iridium-Catalyzed Enantioselective Allylic Substitution with Aqueous Solutions of Nucleophiles
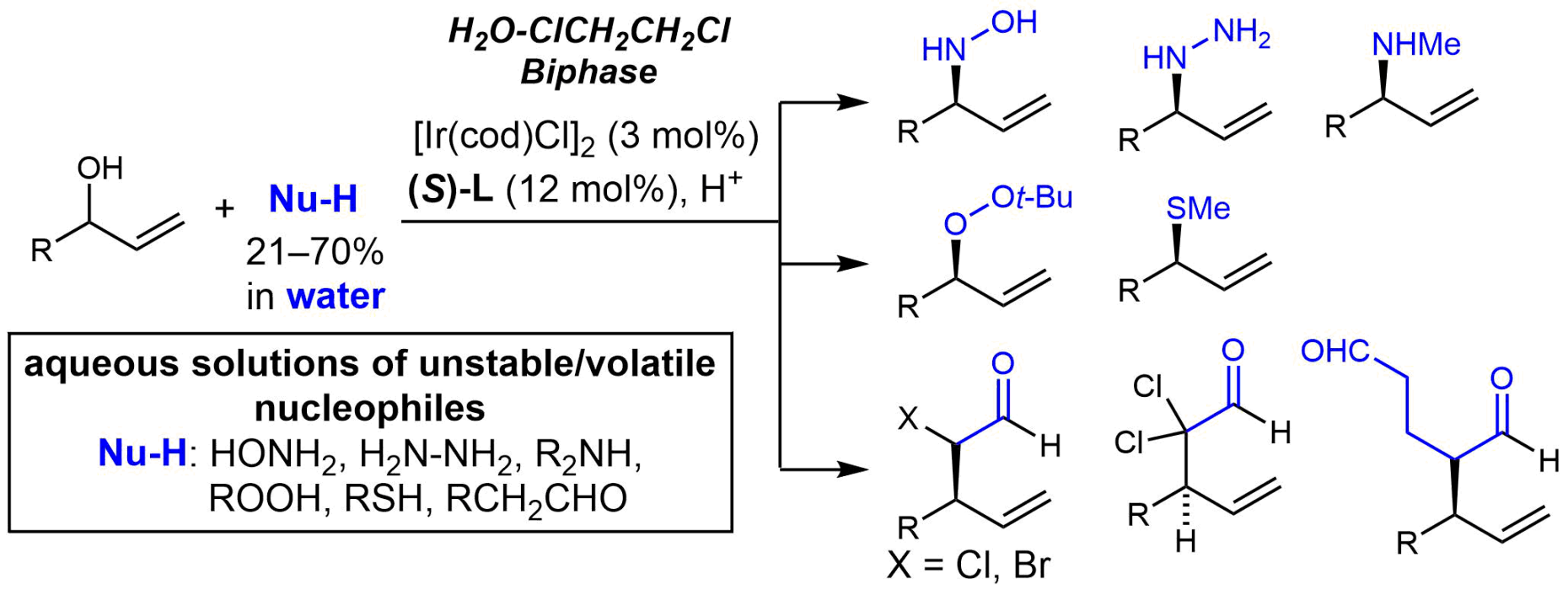
For more information, check out the following publication:
Palladium-Catalyzed C–H Alkynylation of Unactivated Alkenes
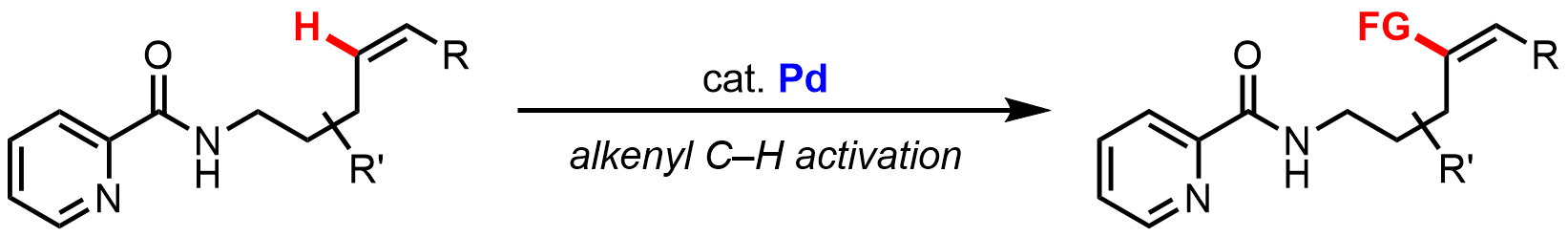
For more information, check out the following publication:
Iridium-Catalyzed Enantioconvergent Allenylic Alkylation:
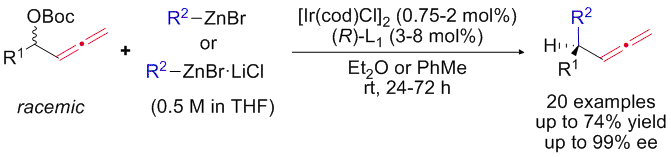
For more information, check out the following publications:
- external page D.A. Petrone, M. Isomura, I. Franzoni, S.L. Rössler, E.M. Carreira, J. Am. Chem. Soc. 2018, 140, 4697-4704.
- external page M. Isomura, D.A. Petrone, E.M. Carreira, J. Am. Chem. Soc., 2019, 141, 4738–4748.
- external page F. Glatz, D.A. Petrone, and E.M. Carreira, Angew. Chem. Int. Ed. 2020, Accepted Article.
Enantio- and Diastereodivergent Dual Catalysis and Its Application to the Synthesis of All Four Stereoisomers of THC:
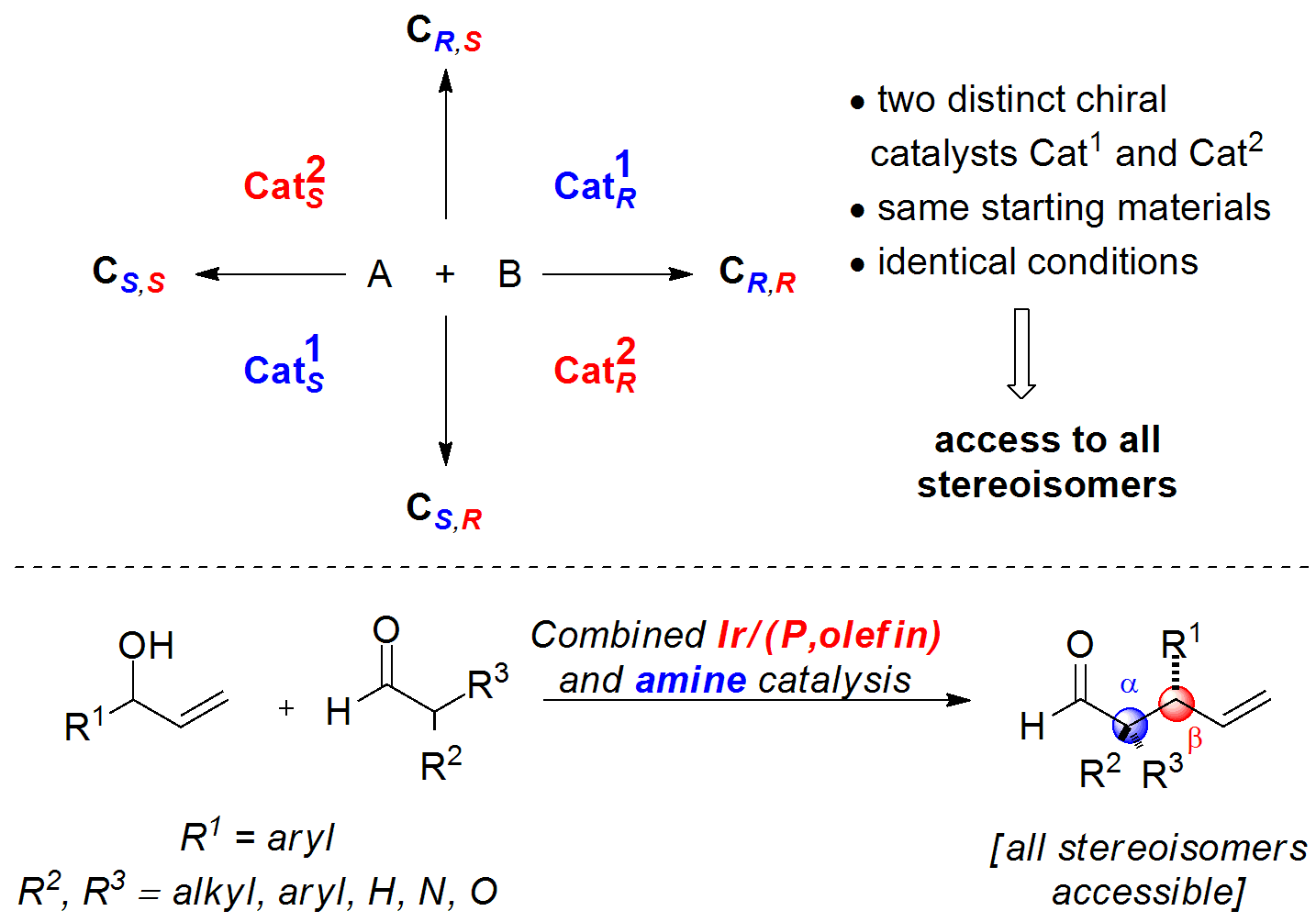
For more information, check out the following publications:
- external page S. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science, 2013, 340, 1065.
- external page S. Krautwald, M. A. Schafroth, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3020.
- external page M. A. Schafroth, G. Zuccarello, S. Krautwald, D. Sarlah, E. M. Carreira, Angew. Chem. Int. Ed. 2014, 53, 13898.
- external page T. Sandmeier, S. Krautwald, H. F. Zipfel, E. M. Carreira, Angew. Chem. Int. Ed. 2015, 54, 14363.
- external page S. Krautwald, E.M. Carreira, J. Am. Chem. Soc. 2017, 139, 5627-5639. (Perspective)
Iridium-Catalyzed Asymmetric Allylic Substitution of Allylic Alcohols:
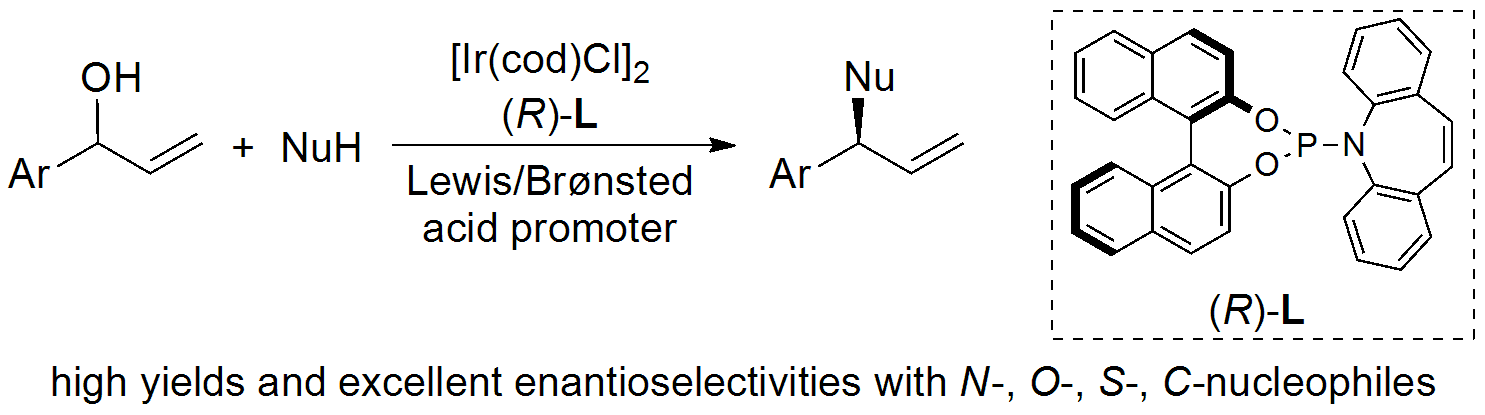
For more information, check out the following publications:
- external page J. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3006.
- external page J. Y. Hamilton, N. Hauser, D. Sarlah, E. M. Carreira, Angew. Chem. Int. Ed. 2014, 53, 10759.
- external page O. F. Jeker, A.G. Kravina, E. M. Carreira, Angew. Chem. Int. Ed.2013,52, 12166.
- external page M. A. Schafroth, D. Sarlah, S. Krautwald, E. M. Carreira, J. Am. Chem. Soc.2012,134, 20276.
- external page J. Y. Hamilton, D. Sarlah, E. M. Carreira, Angew. Chem. Int. Ed.2013,52, 7532.
- external page J. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc.2013,135, 994.
- external page M. Lafrance, M. Roggen, E. M. Carreira, Angew. Chem. Int.Ed.2012,51, 3470.
- external page S.L. Rössler, S.Krautwald, E.M. Carreira, J. Am. Chem. Soc. 2017, 139, 3603-3606.
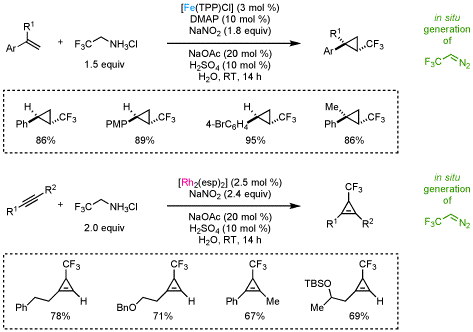
Generation of Trifluoromethyl Cyclopropanes and Cyclopropenes:
For more information, check out the following publications: